The production, use, and disposal of nuclear materials leads to the inevitable release of radioactive compounds into the environment. There are many methods for cleaning up such contaminants via immobilization (via reducing them to insoluble phases) or crystallizing them into stable minerals (actinide incorporation). My research encompasses a broad spectrum of techniques to evaluate the behavior and mitigation of actinide group elements in natural aqueous environments. These methods include computational modeling, electrochemistry, scanning electron microscopy (SEM), Auger electron microscopy (AES), atomic force microscopy (AFM), and synchrotron x-ray absorption spectroscopy (XAS) performed at Argonne National Laboratory.
Actinide transport in the environment
Benjamin Gebarski
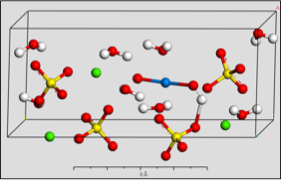
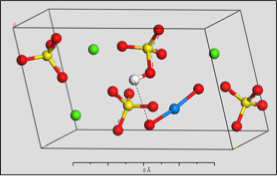
PuO2+ Incorporation Simulation
Using computer models to simulate the incorporation of plutonium into common minerals as they grow.
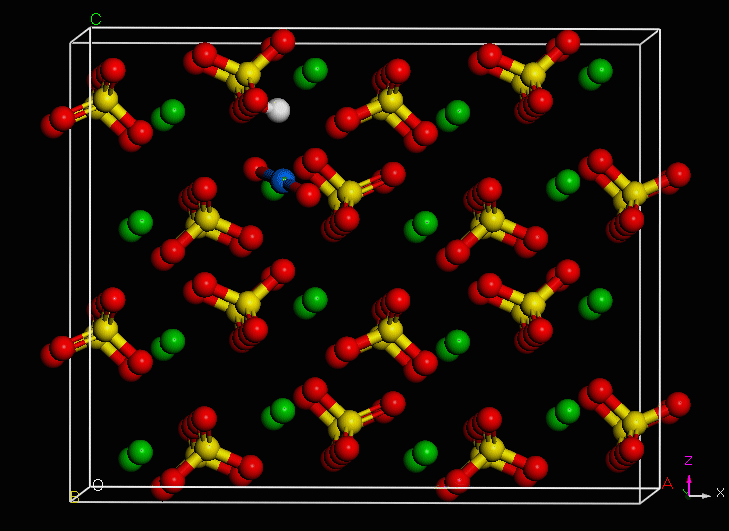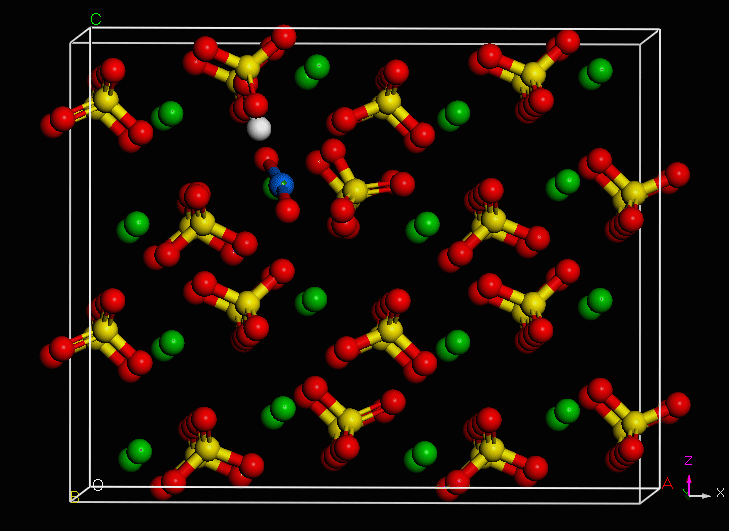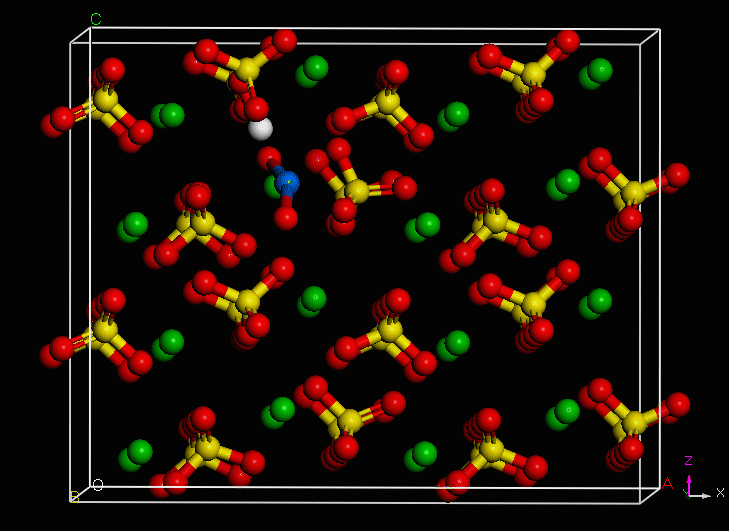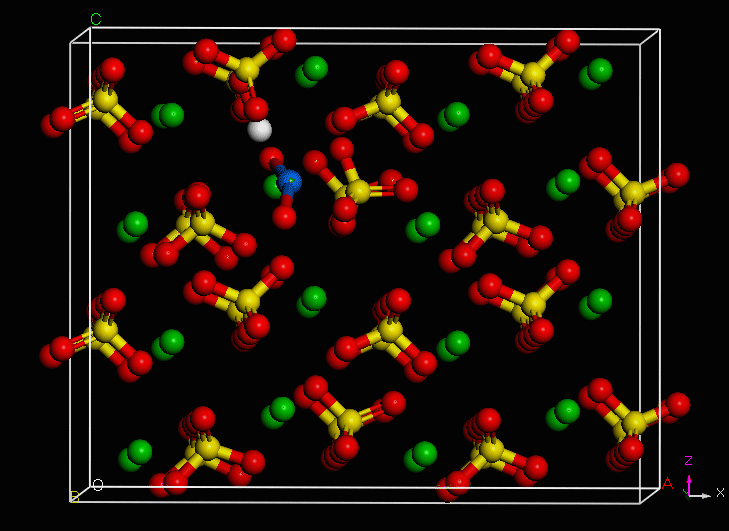

Use of an environmentally-relevant ab initio computational methodology accounting for hydration and surface interaction demonstrates that in laboratory experiments, we will primarily see incorporation proceeding before the system has reached equilibrium by the host mineral actively growing to envelop plutonyl contaminants. If the mineral crystal is allowed to continue growing, we find the PuO2+ can become incorporated in the mineral bulk with the input of minimal energy, which is due to the high-energy dislocation formed by the actinyl in the crystalline structure. Furthermore, we find that based on host minerals tested, that carbonates are more favorable for PuO2+ sequestration than sulfate hosts. These results closely mirror those of experiments with NpO2+ (an analog for the more dangerous PuO2+).
Auger Electron Spectroscopy (AES)
Uranium standards can be compared with experimental samples to ascertain oxidation state, structure, composition, etc.
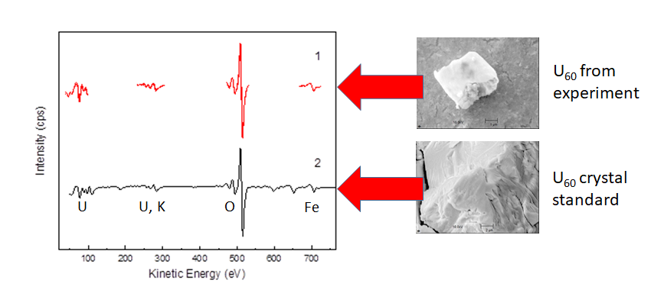
AES is used to visualize and identify sorbates on the substrate surface. Novel uranium compounds are identified and characterized using an AES sensitivity factor, which was derived from the comparison of uranium standards with AES results.
Scanning Electron Microscopy (SEM)
SEM and EDX (energy dispersive x-ray spectroscopy) provide high-resolution imagery and elemental analysis
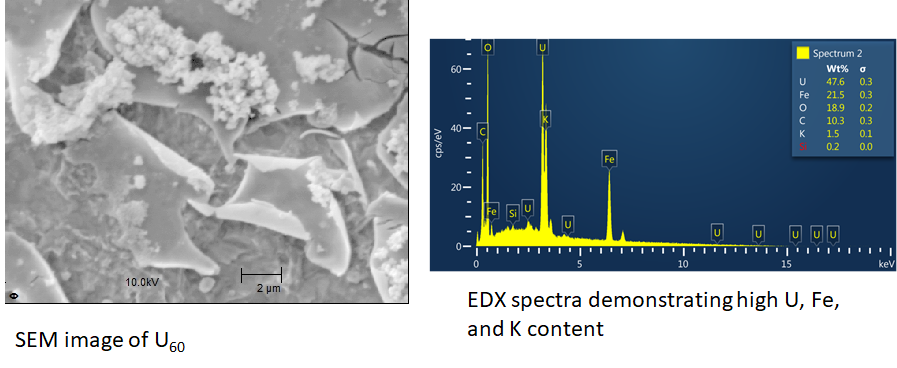
SEM is used primarily to identify, image, and characterize uranium compounds adsorbed to surfaces, packed into electrodes, and precipitated from solution. High resolution SEM (below 100nm) a relatively recent development, can aid in the discovery of uranium compounds completely unique to existing literature.
Electrochemical atomic force microscopy (EC-AFM)
Real-time visualization of electrochemical/redox reactions in situ at substrate surfaces.
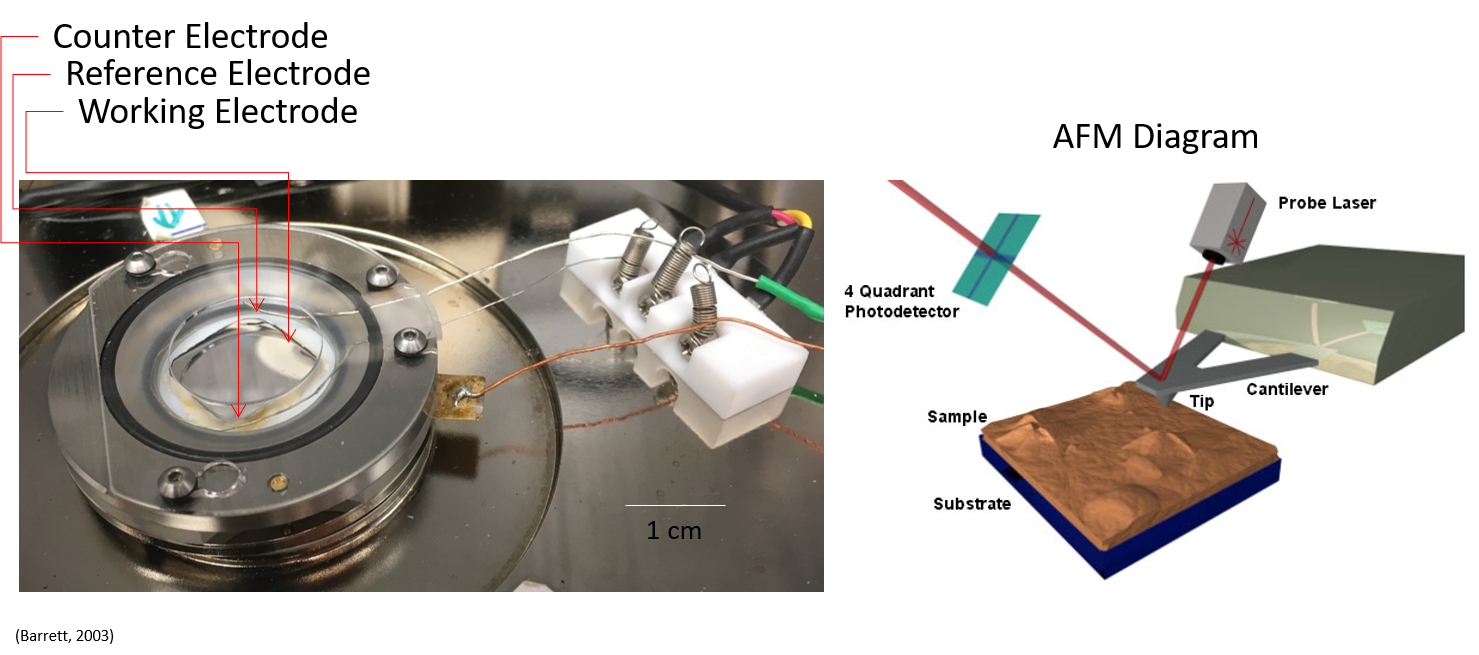
The unique perspective of EC-AFM allows us to visualize both conventional and synthetic uranium compound interactions with a substrate surface as a function of the applied potential. Differences in redox conditions as dictated by the electrode leads to observations of unique U crystals, clusters, and plays a crucial role in U sorbate formation. In this study we demonstrate the feasibility of imaging the entire Eh-pH range of uranyl peroxide reduction products in situ on surfaces.
Synchrotron X-Ray Absorption Spectroscopy (XAS)
To monitor in situ changes in the electronic and structural properties as a function of the applied potential.
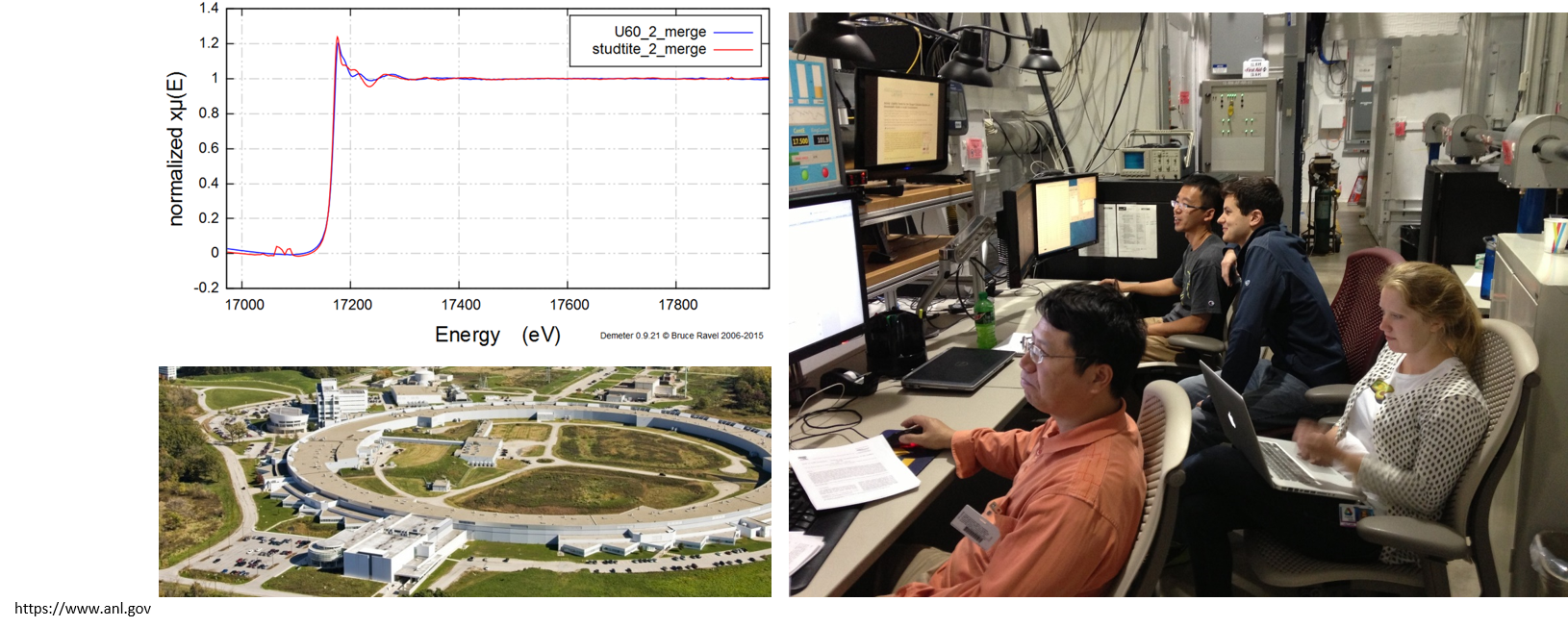
Our work at Argonne National Lab’s Advanced Photon Source began with the design of a custom in situ electrochemical cell with a solution injection reservoir, allowing for the measurement of the thermodynamics and redox potential of the redox switching in uranyl peroxide materilas as a function of pH, solution chemistry, and pe/Eh. Changes in molecular structure, in electronic structure, and the kinetics of growth were monitored by XAS modes (XANES and EXAFS). In situ electrochemical XAS refers to performing electrochemical experiments under operating conditions of the electrochemical cell, i.e., under potential control. This is opposed to performing experiments ex situ, in the absence of potential control. Potential control preserves the electrochemical environment essential to maintain the double layer structure intact and the electron transfer reactions occurring at that particular potential at or near the electrode/electrolyte interface.
Gebarski, B. and Becker, U. (2020) Quantum-mechanical determination of the incorporation of pentavalent plutonium into carbonate and sulfate minerals. Geochimica et Cosmochimica Acta 269, 693-710. https://doi.org/10.1016/j.gca.2019.10.015
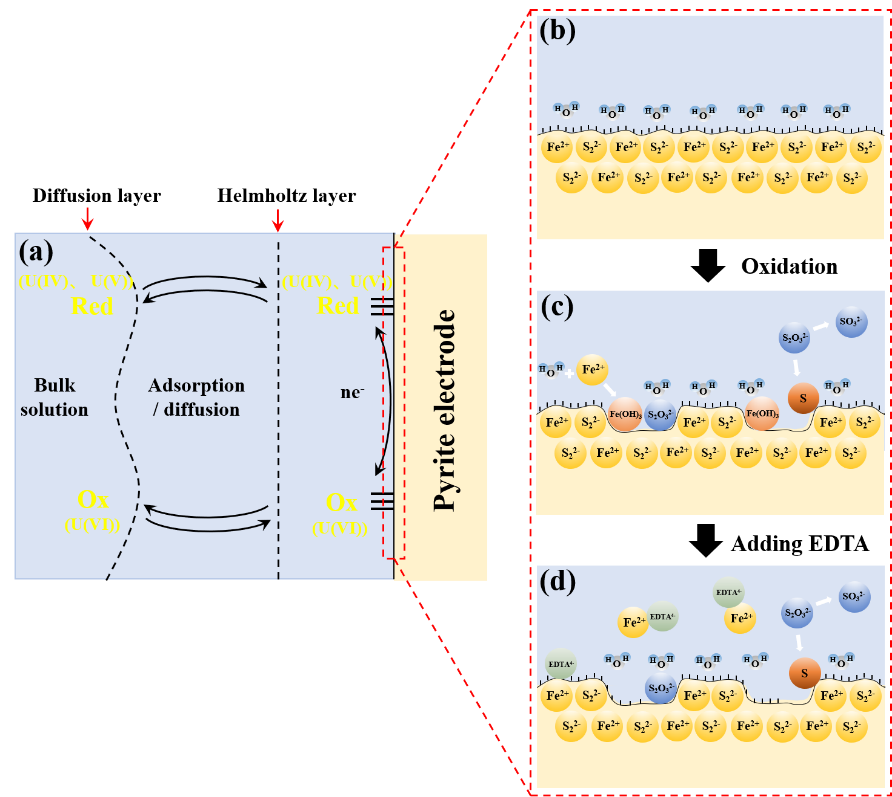
Effect of EDTA Complexation on Redox Reactions of Uranium Mediated by Mineral Surfaces
Yingwei Wang
Ethylenediaminetetraacetic acid (EDTA), a strong complexing agent, can enhance the mobilization of uranium by complexation in the environment. Also, it can affect the redox transformations of uranium species. My research focuses on the effect of EDTA complexation on redox reactions of uranium at the mineral-solution interface. In my research, The powder microelectrode technology was involved to evaluate uranium redox processes occurring in situ under various solution conditions. We can control the rate of reaction of uranium species by changing the scan rate of the electrochemical potential. Finally, the process mechanism of uranium redox occurring in the heterogeneous reaction interface involving the effect of EDTA was proposed.








Multistep Reduction Kinetics Calculation of Actinyl-EDTA Complexes
Sooyeon Kim
The reduction of actinyls to relatively insoluble forms is decreased when they are complexed by inorganic, such as carbonates, or organic ligands, such as ethylenediaminetetraacetic acid (EDTA). Our previous study by Bender et al. [1][2] divided the reduction process of uranyl (or uranyl-tricarbonate complex) by reductants into four sub-steps to determine the reaction rates of each step and which one is the rate determining step. My research aims to get kinetic data sets of the reduction reaction of actinyl-EDTA complex by a reductant (Fe2+) using the same method as described in Bender et al. The principle is to approach the actinyl-EDTA and a reductant in small incremental distances and calculate the system energy at each distance using quantum-mechanical calculations.
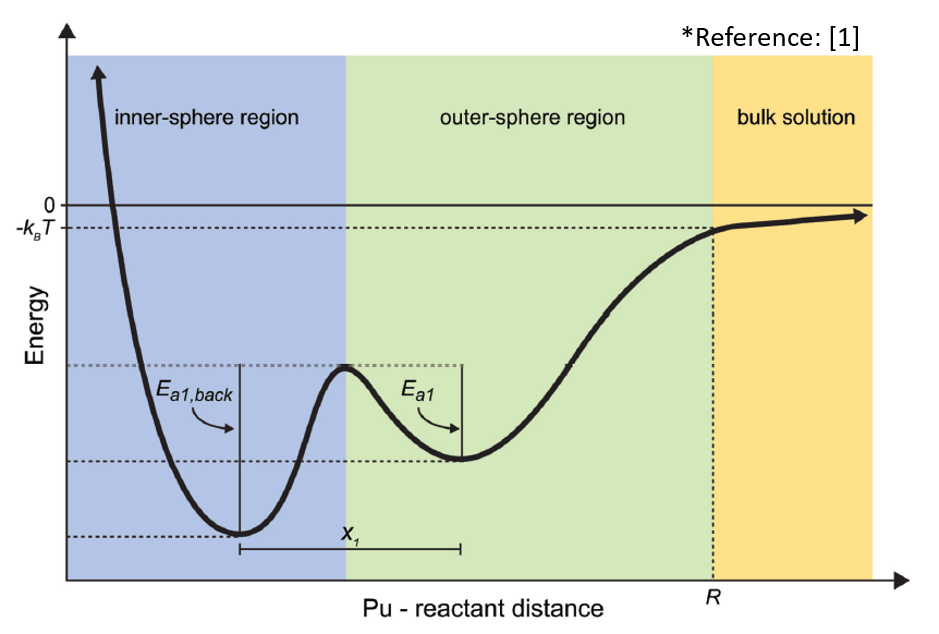








[1] Bender, W.M. and Becker, U. (2018) Determining the kinetics of discrete aqueous redox reaction sub-steps using computational methods: application to reactions of plutonyl (PuO2+/2+) with Fe2+, Fe3+, and hydroxyl radical (•OH), American Journal of Science 318(9), 893-920. DOI: 10.2475/09.2018.02
[2] Bender, W.M. and Becker, U. (2019) Resolving the kinetics of individual aqueous reaction steps of actinyl (AnO2+ and AnO22+; An = U, Np, and Pu) tricarbonate complexes with ferrous iron and hydrogen sulfide from first principles. Radiochimica Acta. https://doi.org/10.1515/ract-2018-3083
Imaging the Reduction of Uranyl on Fe-bearing Minerals Surfaces Using in Situ Electrochemical AFM
YoungJae Kim
Semiconducting solids, such as magnetite (Fe3O4) and ilmenite (FeTiO3), can facilitate otherwise (for homogeneous reactions in solution) slow electron transfer between a reductant and oxidant as these species are adsorbed on their surfaces. This can happen by electron shuttling through their near-surface region of by aiding in the dehydration or decomplexation of these complexes. By using in-situ AFM analysis combined with electrochemical methods, we are imaging reduction of uranyl in the presence of organic ligands such as EDTA and oxalate. The synchronous microscopic and electrochemical data suggest that reduction of uranyl-organic complexes can be facilitated by the catalysis of Fe-bearing minerals.
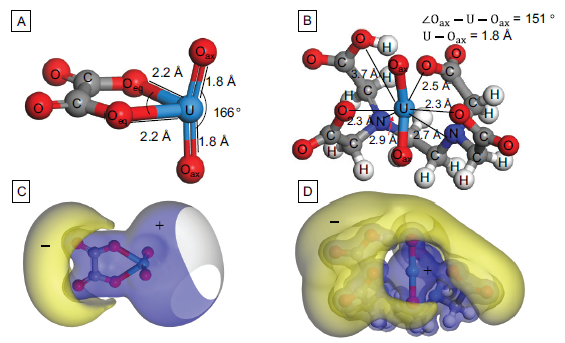








Energy-optimized structures and electrostatic potential surfaces of [(UO22-)(oxalate)]0 (A, B) and [(UO22-)HEDTA]- (B and D). In figures 3C and 3D, the blue and yellow surfaces represent positive and negative potentials, respectively.
Mechanistic Study of Wettability Change on the Calcite Surface
Sooyeon Kim
Polar compounds of crude oil have a significant influence on binding hydrophobic molecules to mineral surfaces. Most of these polar compounds are in the resin or asphaltene fraction of crude oil and having various functional groups in their complex and high aromaticity structures. Among the various functional groups, I am focusing on the alcohol functional group, which is an identified functional group in asphaltenes, to understand how the interactions between hydroxyl groups in asphaltenes and mineral surfaces begin. Adsorption energies and bonding structures of oil components with the alcohol functional group are investigated using DFT. And the dynamic properties of oil molecules are studied using classical MD.








Kim S., Marcano M.C., and Becker U. (2019) Mechanistic study of wettability changes on calcite by molecules containing a polar hydroxyl functional group and nonpolar benzene rings. Langmuir 35(7), 2527-2537. https://doi.org/10.1021/acs.langmuir.8b03666
My research aims to understand mechanisms controlling uranium transport in the subsurface. One way is to reduce U(VI) (soluble in water, thus easily transported in groundwaters systems) to U(IV) (low solubility leading to precipitation of uranium phases). The abiotic, homogeneous reduction of uranium by reductant such as Fe(II) is debated, some studies report it is kinetically inhibited while others suggest it is a function of reductant concentrations, pH, etc. Electron transfer calculations on the homogeneous reduction system are carried out using ab initio methods to gain insight as to whether the process is kinetically favorable and what factors may influence the rate. The heterogeneous reduction of U(VI) by Fe(II) in the presence of an iron oxide substrate (i.e. hematite) has been shown to be thermodynamically and kinetically favorable.
I conduct batch coadsorption experiments on the coadsorption of U(VI) and Fe(II) to hematite and corundum to investigate whether how the minerals electronic properties may affect these redox processes; i.e. does hematite as a semiconductor facilitate reduction of uranium over its insulating, isostructure corundum? The aqueous fraction is characterized using ICP-MS and UV-Vis while the solid fraction is characterized using XRD, AES, and XPS. In addition to these experiments electron transfer calculations are also conducted on these ternary systems for the first time. Through combining experimental and computational results the influence of minerals and their properties can be better understood.
Uranium transport in the subsurface
Sandra Fernando








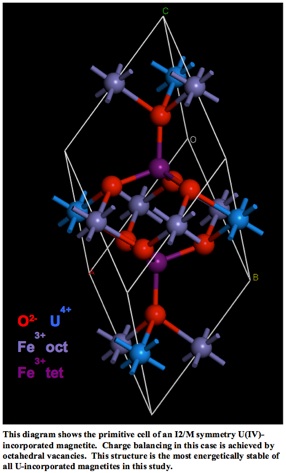
Currently, my research is focused on understanding actinide elements incorporation into environmentally relevant oxide phases, particularly the iron oxide magnetite (FeFe2O4). I have been employing ab initio quantum mechanical simulations to study the energetics of such incorporation reactions and the structure of incorporated sinks. Recent efforts have centered on reactions involving a magnetite sink. The possibilities for incorporation are more diverse in magnetite when compared to hematite. Magnetite has two iron oxidation states (ferric and ferrous) and two structural sites (tetrahedral and octahedral) present in its structure. While uranium and other large actinide species prefer the octahedral site, charge balancing may be achieved by vacancies in either the tetrahedral or octahedral site. Additionally, spin state considerations must be taken in to account as both ferric and ferrous iron have unpaired spins, as does U(IV).
The initial investigations have considered reactions involving solid sink and source phases. Energies of periodic solids have been carried out using CASTEP code employing the generalized gradient approximation (GGA) and PBE exchange-correlation functional. Some examples of these solid phase reactions include the following for U(IV) and U(VI) incorporation into magnetite:
UO2 + Fe2Fe4O8 Fe2UFe2O8 + 2 FeO
3 UO2 + O2 + 2 Fe2Fe4O8 3 Fe2UFe2O8
UO3 + Fe2Fe4O8 FeUFe3O8 + Fe2O3 (U6+ in oct. site, vacant Fe3+ oct. site)
UO3 + Fe2Fe4O8 Fe2UFe2O8 + Fe2O3 (U6+ in oct. site, vacant Fe3+ tet. site)
Note: In the second reaction, diatomic oxygen is treated as a single molecule in periodic boundary conditions with P1 symmetry.
While these solid phase incorporation reaction energies are relatively straightforward to calculate, they are also not the best representation of real processes. It is much more likely that source cations are present as aqueous complexes and incorporation reactions occur at the solid-liquid interface, and only after a series of sorption and surface precipitation processes. To expand on this research it will be important to consider all possible steps involved to develop a more complete picture of these reactions. To do this we will utilize different computational methods involving a combination of periodic solid and cluster calculations.
Computational modeling of U(VI) and U(IV) incorporation in to magnetite (FeFe2O4)
Will Bender








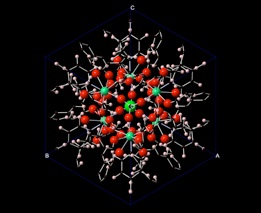
Development of micro- and meso-porous actinide materials in recent years has demonstrated their enhanced potential for application for nuclear waste separations. However, besides their structural characteristics, very limited data on their chemical and physical properties are available. The electronic and thermodynamic properties of metal-organic compounds are studied from ab-initio methods. Using high-level density functional theory (DFT) and combining cluster and periodic approaches, we have recently developed a method for calculating the energetics of charged actinyl incorporation into periodic solids. This method allows us to analyze the incorporation reaction energetics in more realistic way where simplified reactions with solid sources and sinks are replaced with realistic aqueous complexes.
We apply this approach to estimate the incorporation limits and selective incorporation of specific actinides, actinyls from aqueous source to actinide containing metal-organic compounds in order to understand the thermodynamics of their selectivity towards actinides, particularly, transuranium elements.
Thermodynamics of selectivity of metal-organic compounds for specific actinide ions
Saumitra Saha








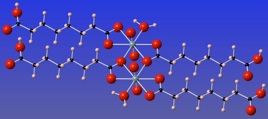
Photochemical and catalytic properties of MOFs
Saumitra Saha
The photocatalytic activities of uranyl containing MOFs involve photo-excitation of electrons and subsequent charge transfer between ligand and metal centers. These are the key steps in photocatalytic degradation of organic contaminants (Corma, Garcia et al. 2010) and the potentially facilitate efficient harvesting of light. The understanding of the mechanism of such processes in MOFs is the key to design new photocatalytic materials. We apply electronic structure calculations to evaluate the energetics of the charge transfer reactions in MOFs.
Studies in our group demonstrated the water-enhanced oxidation of UO2 (Skomurski, Shuller et al. 2008). Other studies have shown the catalytic role of multi-valent actinide oxides in facilitating water dissociation (Haschke, Allen et al. 2000). The role of radiolytic redox-active species such as H2O2 and radical OH in the redox-reactions of uranyl and uranyl-based spent fuel is also well studied (Rouyer, Poulesquen et al. 2009). Our goal is to evaluate the role of different combinations of MOFs, radiolysis products, and polarizing species in catalyzing redox reactions and in actinide-containing MOFs undergoing redox transitions themselves. In our previous work, we have shown than in such reactions, spin transfer from or to the reactant (e.g., O2,triplet →O2,singlet or U6+singlet→U4+triplet) can be a major rate-limiting step (Renock and Becker 2010) along with the dissociation of the actual reductant or oxidant. Since computational approaches can resolve individual rate-limiting steps, we study the mechanisms of successive rate-limiting steps in catalytic reactions involving actinide-containing MOFs and identifying the role of MOFs in lowering reaction barriers.








I study actinide elements in the environment through the use of computer modeling and computational chemistry. Currently I'm modeling the interaction of Pu(VI)O2 and it's hydration sphere with common radiolysis products such as HOOH and OH radical using ab initio calculations to determine their effect on the reduction and sorption of plutonium on quartz surfaces.
In the past I have performed bulk analysis on andesites using the electron microprobe to determine closing temperature and the water content in plagioclase crystals.
Reduction and sorption of plutonium on quartz surfaces
Evan Killeen
Analysis of uranyl peroxide nanoclusters using cyclic voltammetry and cavity microelectrodes to augment induced potential to oxidize/reduce clusters, identifying them in reference to standard electrode potentials investigating redox potentials as a function of pH, background solution, mineral surface, or electrolyte. These conditions can describe how actinide species in environmental conditions interact with mineral surfaces and surface-mediated redox processes. Such information is essential to any experiment concerning sorption, precipitation, diffusion, incorporation, surface complexation, and solid solution formation
Analysis of uranyl peroxide nanoclusters
Ben Gebarski
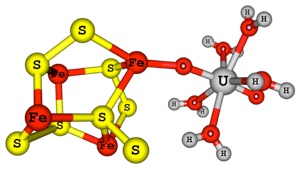
Redox active and semiconducting minerals can promote a range of chemical reactions in their surfaces. The chemistry and mechanisms of surface mediated processes at molecular level detail is still lacking and not properly understood. For instance, iron sulfide minerals such as pyrite and mackinawaite are the most abundant minerals on the earth’s crust.
Reduction of actinyl ions have been reported on pyrite and mackinawaite minerals. However, the mechanisms of reduction are poorly understood. Depending on the mineral surface different end products were speculated. This asks for computational chemists to propose various possibilities and pathways for redox process on mineral surfaces and based on the computed free energy values for the proposed mechanisms with appropriate computational approach, a viable and most probable pathway will be elucidated.
Classical, ab initio and DFT based methods will be utilised according to the nature of the problem. Cluster model approach will be employed to investigate the reactions taking place on these mineral surfaces, in particular redox process of radioactive actinide nuclear wastes. In addition, reduction, precipitation and retention process of hazardous elements (Tc, Cr, As and Se) and their interactions with these minerals surfaces will be an extension of this study. Cluster model geometry for pyrite-uranyl ion interaction is shown in Figure. 1
Surface Promoted Redox Process of Actinides
Krishnamoorthy Arumugam








Computational modelling of plutonyl and neptunyl incorporation onto carbonate and sulfate mineral surfaces. To understand these processes at the molecular level, ab-initio cluster-model computational approaches are applied and compared to existing experimental results. These models are used to propose incorporation energy, dissociation, redox chemistry, and disproportionation mechanisms. Heterogeneous electron transfer processes between sulfate or carbonate mineral surfaces and plutonyl and neptunyl are investigated using kinetic and thermodynamic models within the Marcus theory of electron transfer framework
Computational modelling of plutonyl and neptunyl incorporation onto carbonate and sulfate mineral surfaces
Ben Gebarski

















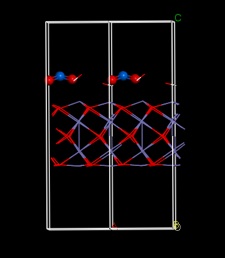








Electrochemical methods provide a unique in situ approach that allows one to investigate redox behavior of intermediate products, such as U(V)O2+ on surfaces of semiconducting minerals. Magnetite (Fe3O4) is found as the corrosion product of iron in anoxic conditions, and has showed it ability to reduce U(VI) to lower oxidation states. Redox reactions of uranyl ions on the surfaces of powder and bulk magnetite are studied by means of cyclic voltammetry and potential step chronoamperometry.
Electrochemical redox reactions of uranyl(VI) on magnetite
Ke Yuan
In order to observe the surface change at different Eh, a series of images of magnetite surface in U(VI) solution were captured by AFM as a function of the applied voltages.
Imaging uranyl(VI) redox reactions on magnetite by in situ electrochemical AFM
Ke Yuan








HfO2 is a neutron absorber and has been mechanically mixed with UO2 in nuclear fuel in order to control the core power distribution. During nuclear fission, the temperature at the center of the fuel pellet can reach above 1300 K, where hafnium may substitute uranium and form the binary solid solution of UO2-HfO2. However, experimental data on the UO2-HfO2 binary are limited. The enthalpy of mixing of the UO2-HfO2 binary with three different structure frameworks were calculated in this study using density functional theory and subsequent Monte Carlo simulations. The free energy and entropy of mixing were obtained from thermodynamic integration of the enthalpy of mixing over temperatures.
Computational modeling of the UO2-HfO2 solid solution
Ke Yuan
The incorporation of radionuclides into mineral hosts may strongly influence the concentration and migration of radioactive contaminants in the subsurface. Because of this potential to sequester radioactive species such as uranyl (UO22+), neptunyl (NpO2+), and radium, I am interested in the thermodynamics and the resulting structures of radionuclide incorporated minerals, which may be directly applicable to the engineering of permeable reactive barriers to impede these elements or to the development of predictive models that calculate the mobility of metal contaminants in surface and near-surface environments.
In one study, I am using density functional theory to calculate the energetics, structure, and electronic configuration of uranyl and neptunyl incorporation into sulfate (gypsum, anhydrite, anglesite, celestine, barite) and carbonate minerals (calcite, aragonite, cerussite, strontianite, witherite). Incorporation reactions involve both solid, periodic mineral phases as well as hydrated ions in solution; therefore, we must use two different types of quantum mechanical models. With a new approach developed by current and former members of our research group, periodic and cluster computational methods are combined as separate chemical equations in which each species is calculated under the same parameters.
In another study, I am looking at the thermodynamic properties of mixing (entropy, enthalpy, and Gibbs free energy) calculated from molecular mechanics for the (Ra,Ba)SO4 solid solution. The natural affinity between radium and barite has remained a topic of interest since the early days of radiochemistry at the end of the nineteenth century, and more recently, the (Ra,Ba)SO4 solid solution has been observed as a by-product in multiple industrial operations, including uranium ore processes, oil production, and phosphoric acid manufacturing. Despite the connection between radium and barite, relatively little thermodynamic or structural data has been published for radium sulfate or the (Ra,Ba)SO4 solid solution. In this study, empirical forcefields are used to calculate the entropy, enthalpy, and free energy of mixing. Preliminary results indicate that the Ra-O interatomic potential is described by a Buckingham potential, though consequential conclusions from the thermodynamic evaluation of the (Ra,Ba)SO4 solid solution are forthcoming.
Radionuclide incorporation and uptake into sulfate and carbonate minerals
Sarah Walker